Human Living-Blood
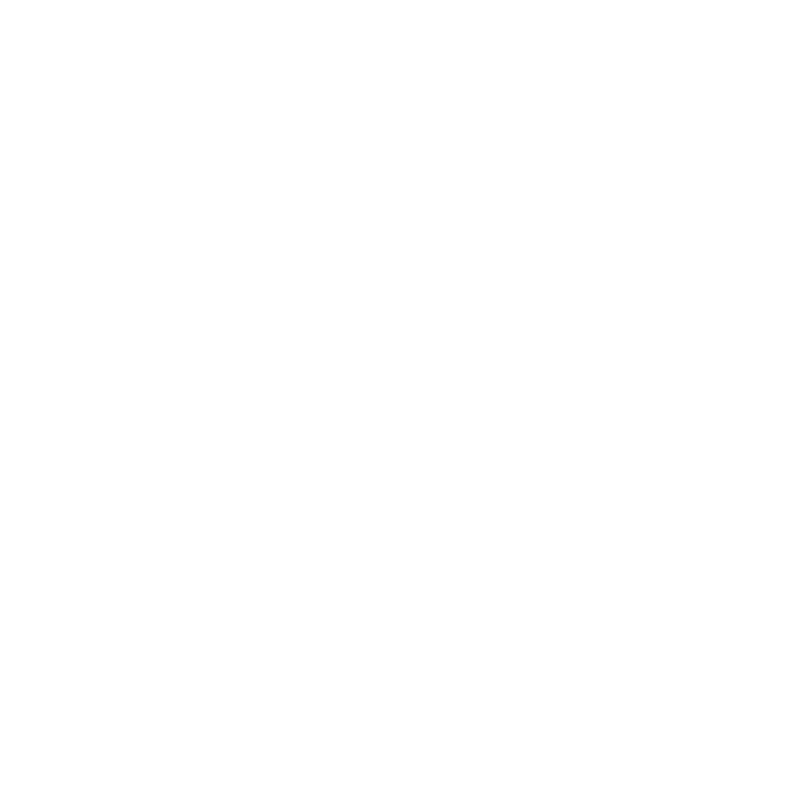
An Isolated System for Assessing Immune Responses
Pebble's HUMAN LIVING-BLOOD Systems
The HUMAN LIVING-BLOOD System is Pebble’s state-of-the-art solution for modelling the human immune response within a controlled ‘phase minus-one’ setting. This extracorporeal platform maintains whole blood at physiological pressures, flows, temperature, and gaseous exchange. The complete blood compartment, including all leukocyte subsets, red blood cells, platelets, cytokines, exosomes, antibody, complement are fully functional.
By accurately simulating human vascular haemodynamics, the LIVING-BLOOD system can analyse immune reactivity, cytokine storms, antibody production, and complement activation, offering an in-depth understanding of the immunogenicity and toxicity potential of new therapies.
Incorporating a modular design, the HUMAN LIVING-BLOOD System provides a holistic view of organ-specific and systemic immune responses. This enables the study of complex interactions within the immune system, crucial for the development of immunotherapies and biocompatible devices.
Blood Evaluation Services
Immune Evaluation
- Evaluating the Impact of Drugs on Innate and Adaptive Immunity
- Mechanistic Studies of Immunomodulatory Agents
- T-cell Modulation by Checkpoint Inhibitors
- Assessment of Drug-Induced Immunogenicity
- Screening for Off-Target Immune Effects
- Profiling Immune Responses to Biologics
- Longitudinal Studies of Immune System Recovery Post-Intervention
- Exploratory Studies for Novel Immunotherapies
- Baseline and Induced Cytokine Release Profiles
- Drug Efficacy Assessment through Cytokine Suppression
- Kinetics of Cytokine Release upon Drug Exposure
- Multiplex Cytokine Assays for Broad Spectrum Analysis
- Correlation of Cytokine Levels with Clinical Outcome
- Detailed Phenotyping of Immune Cell Subsets
- Functional Capacity of Neutrophils and Antigen-Presenting Cells
- T-cell Subsets Functionality under Drug Influence
- Natural Killer (NK) Cell Activation and Target Killing Efficiency
- Impact of Immunosuppressive Agents on Cell-Mediated Immunity
- Kinetics of Antibody Production Post-Exposure to Antigens
- Profiling of Drug Effects on Antibody Isotypes and Subclasses
- Assessment of Neutralizing Versus Non-neutralizing Antibody Responses
- Monitoring of Autoantibody Production for Autoimmune Potential
- Detailed Cascade Assays for Each Complement Pathway
- Measurement of Membrane Attack Complex (MAC) Formation
- Screening of Complement Inhibitors and Regulators
- Assessment of Complement-mediated Opsonization and Phagocytosis
- Organ-Specific Immune Response to Pathogen Mimics
- Drug Efficacy in Modulating Organ-Specific Inflammation
- The Role of Organ-Specific Immune Cells in Disease Models
- Modelling Organ-Immune Interactions in Autoimmune Diseases
- Panel of Acute and Chronic Inflammatory Markers
- Time-Course Analysis of Inflammatory Responses
- Biomarker Correlation with Organ-Specific Injury or Repair
- Validation of Novel Inflammatory Biomarkers for Clinical Relevance
- Migration Assays under Static and Flow Conditions
- Impact of Drugs on Endothelial Activation Markers
- Visualization and Quantification of Leukocyte-Endothelial Interactions
- Studies on the Modulation of Endothelial Barrier Function
- Modelling of Immune System Response to Biomaterials
- Development of Protocols for Induction of Immune Tolerance
- Evaluation of Drug Efficacy in Autoimmune Disease Models
- Analysis of Immune Response to Novel Therapeutic Constructs
- Development of Bespoke Immune Challenge Models
- Real-time Monitoring of Immune Response to Therapeutics
- Personalized Immune Profiling for Targeted Therapy Development
- Post-Treatment Immune System Reconstitution Studies

Platelets & Red Blood Cells
- Measurement of platelet aggregation and adhesion.
- Assessment of platelet activation markers post-drug exposure.
- Monitoring platelet lifespan in response to therapeutics.
- Analysis of platelet interaction with vascular endothelium.
- Evaluation of drug effects on platelet granule release and content.
- Investigating the role of platelets in drug-induced thrombosis.
- Studying platelet function in the context of antiplatelet therapy.
- Quantifying the effects of drugs on platelet morphology and structure.
- Profiling platelet-mediated immunomodulatory effects.
- Assessing the impact of drug candidates on platelet-derived growth factors.
- Evaluation of drug-induced changes in RBC membrane integrity.
- Measurement of erythrocyte deformability.
- Monitoring of haemoglobin content and oxygen-carrying capacity.
- Assessment of RBC survival under therapeutic stress.
- Analysis of RBC aggregation and its impact on microcirculation.
- Investigating drug effects on erythropoiesis and RBC maturation.
- Evaluation of oxidative stress markers in RBCs post-drug exposure.
- Monitoring the effect of drugs on RBC metabolism.
- Assessment of drug interaction with RBC transporters and ion channels.
Profiling of RBC responses to haemolytic agents.
- Detailed co-oximetry evaluations for oxyhaemoglobin and methaemoglobin levels.
- Assessment of carboxyhaemoglobin to evaluate potential carbon monoxide exposure.
- Analysis of haemoglobin derivatives and their functional implications.
- Blood gas analysis including pO2, pCO2, and pH measurements.
- Evaluation of bicarbonate and total CO2 content in the blood.
- Analysis of electrolyte levels, including sodium, potassium, calcium, and chloride.
- Lactate measurements to assess metabolic status under drug influence.
- Examination of the buffering capacity of blood through base excess/deficit calculations.
- Assessment of arterial and venous blood gas differences for circulatory assessments.
- Profiling of blood gas parameters to monitor respiratory and metabolic function under various therapeutic conditions.
Standard Drug Development Tests
- Evaluating antigenicity potential and T-cell epitope mapping.
- Determining the risk of anti-drug antibody (ADA) development.
- Investigating the propensity for drug-induced autoimmunity.
- Characterising the specificities of ADAs and their clinical implications.
- Assessing conformational stability and post-translational modifications that may affect immunogenicity.
- Analysing the impact of drug administration routes on immune response.
- Investigating B-cell memory and long-term immunogenic effects.
- Assessing potential for immunogenicity in diverse genetic backgrounds using polymorphic immune model systems.
- Evaluating the effect of immune adjuvants on drug response.
- Profiling the maturation of the immune response over time post-drug exposure.
- Evaluating the impact of therapeutics on immune cell life cycle and turnover.
- Investigating the effects of drugs on immune tolerance mechanisms.
- Profiling the impact of therapeutic agents on cytokine networks.
- Assessing the impact of drugs on immune cell receptors and signalling pathways.
- Investigating the potential for drugs to induce immunosuppression and affect vaccine responses.
- Evaluating the effect of therapeutics on the differentiation and function of immune cell subsets.
- Studying drug effects on the crosstalk between the immune system and microbiome.
- Assessing the potential for drugs to cause immune-related organ damage.
- Profiling the immune system before and after drug-induced stress responses.
- Investigating immune cell metabolism and bioenergetics in response to therapeutic agents.
- Establishing safety margins with comprehensive immune system profiling.
- Identifying and characterizing novel immune-related toxicity biomarkers.
- Evaluating the reversibility of drug-induced immunological effects post-exposure.
- Profiling immune system resilience and compensatory mechanisms post-drug exposure.
- Assessing the risks of drug-induced immunopathology across different tissues.
- Characterizing the kinetics of immune system recovery following drug withdrawal.
- Evaluating long-term effects of drug exposure on immune memory and function.
- Studying the impact of drug formulations and metabolites on immune health.
- Establishing thresholds for immunotoxicity across a range of therapeutic dosages.
- Assessing the potential for synergistic immunotoxic effects in combination therapies.
Precision Immune Analysis
Accelerating to Clinic
Pebble’s HUMAN LIVING-BLOOD System is designed to align with the requirements of regulatory authorities, such as the FDA, supporting the safe transition from preclinical testing to clinical trial.
Pebble provides tailored protocols that fit within the acute phase of data collection, and the system generates crucial early-stage immunogenicity and safety insights. These support subsequent stages of the drug approval process, offering data that underpins the potential clinical success of therapeutic candidates. This ‘phase-minus one’ approach is unique to Pebble.

Immunogenicity Risk Assessments
Evaluate antigenicity potential & T-cell epitope mapping, fitting within a 48-hour time frame for acute phase data collection.
Anti-Drug Antibody Assessments
Risk of ADA development can be assessed, crucial for early preclinical immunogenicity screening
Drug-Induced Autoimmunity
We can evaluate drug-induced autoimmunity & characterising ADA specificities during the early phase of the immune response
Immunotoxicology Profiling
Supports immunotoxicology profiling, including immediate effects on immune cell life cycles & turnover within the acute exposure period.
Early Safety & Toxicology Endpoints
We provide early-phase safety & toxicity endpoint establishment through comprehensive immune system profiling & identification of toxicity biomarkers.
Innate & Adaptive Immunity
We offer a controlled setting for immuno-pharmacology studies, evaluating the drug's impact on innate & adaptive immunity, aligning with FDA’s focus on mechanistic studies.

Immunological Safety Evaluation
We use validated SOPs to replicate human haemodynamics & immune reactions, collecting data relevant for FDA's criteria for preclinical immunological safety evaluation.

Cytokine Profiling & Analysis
Provides cytokine profiling & analysis immediately upon drug exposure, adhering to FDA guidance for early evaluation of cytokine storm risk.
Find Out More
Want to learn more about the advantages of our Human LIVING-BLOOD Systems? Sign up with your email below to access an exclusive resource.

