Transplant Studies
A Novel Platform for Transplant Research, Fast-tracking Innovations to Clinic
The Pebble LIVING-ORGAN system replicates in-vivo physiology by circulating warm, nutrient-rich, oxygenated blood through arteries, and deoxygenated, nutrient-depleted blood through veins. Entire porcine organs are connected to a clinical life-support system which mimics vascular anatomy, so physiological blood supply, biochemistry and organ function are restored.
Perfusion Protocols
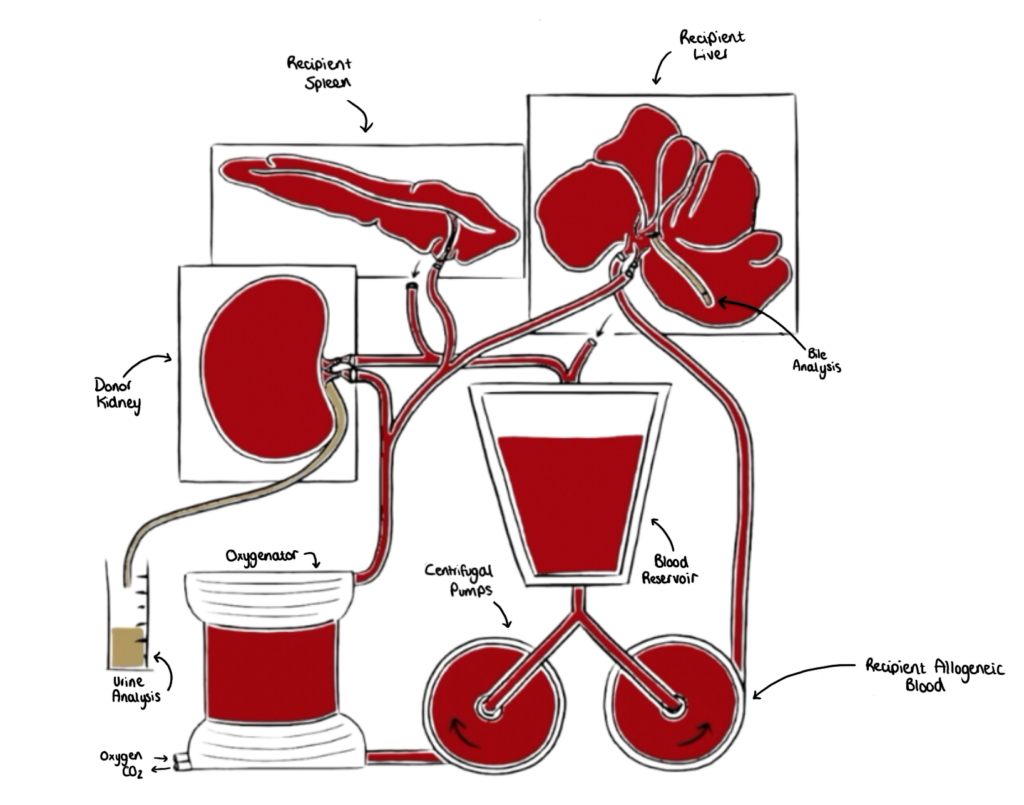
Organs are perfused with autologous or allogeneic blood to model the donor or recipient.
Our allogeneic transplant-reperfusion system recreates transplantation by connecting a donor organ to a LIVING-ORGAN system, consisting of blood and organs from a recipient animal. We replicate aortic and IVC blood flow, with oxygenated arterial blood and deoxygenated venous blood. Transplant reperfusion can then be evaluated in rich detail.
We only use clinical gold standard protocols for organ preservation (including approved cold-storage and machine perfusion approaches). All solutions, consumables and hardware are FDA/MHRA/EMA approved.
Pebble have performed perfusion studies for over 20 years, with more than 1,200 procedures to date – our expert multidisciplinary team are world-leading in this area.
Pebble’s LIVING-ORGAN systems are complex, for more information watch this video:
Transplant Evaluation Services

Transplant Models
- Normothermic machine perfusion (NMP) recirculates autologous blood through an organ at body temperature, with oxygen and nutritional support, which restores organ function and metabolism.
- Pebble has a selection of life-support systems (including commercial centrifugal pumps, roller pumps, peristaltic pumps), combined with an established supply chain utilising a broad range of global critical care companies.
- Hypothermic machine perfusion (HMP) involves perfusing a cold preservation solution through the organ or tissue, with or without, oxygen supplementation. We replicate all HMP protocols in clinical use.
- We follow clinical protocols for static cold storage and only use EMA/FDA/MHRA approved preservation solutions and systems.
- Novel medical devices, preservation solutions, solution additives, protocols and methods of storage can be compared to clinical, regional preservation approaches.
- Our physiological LIVING-ORGAN systems accurately recreate the immediate transplant period. Circuits are loaded with allogeneic blood and organs from a ‘recipient’, and a ‘donor’ organ is connected and reperfused, mimicking immediate post-transplant haemodynamics
- Medical devices, solutions and therapeutics (i.e. novel preservation approaches, donor organ manipulation, immunomodulation and immunosuppression) can be evaluated in significant detail.
- An allogeneic circuit is prepared, using a blood type O recipient, and a blood type A donor organ is then connected. This initiates an antibody mediated rejection (ABMR) response with the hallmarks of clinical ABMR.
- Therapies can be delivered during preservation, machine perfusion, or directly into the ABMR LIVING-ORGAN system.
- The Allorecognition LIVING-ORGAN system is designed to dissect donor-recipient immune system interactions in the immediate post-transplant period. The circuit is created using whole blood and organs from a female ‘recipient’ pig. A male ‘donor’ organ is then connected to the circuit.
- This enables identification and tracking of Y chromosome positive graft leukocytes within the recipient anatomy, including the lymph nodes. Donor leukocyte tracking serves as a direct marker for direct allorecognition.
- In the Graft Infiltration LIVING-ORGAN system, a male recipient and a female donor organ are used. This allows evaluation of graft infiltration, focusing specifically on recipient T cells as they interact with the donor tissue.
- Novel therapies can be supplemented into the circuit, and graft acceptance, immunosuppression, or immunomodulation can be monitored in real-time. This aspect of the ‘Allorecognition’ platform plays a critical role in the development and refinement of therapies.
Organ Specific Functional Assessment
- Glomerular Filtration Rate (GFR): Measurement of GFR is crucial for assessing kidney function, estimated by clearance of insulin or creatinine.
- Serum Creatinine Levels: Creatinine is a waste product filtered by the kidneys; its serum levels are a standard marker of kidney function.
- Urinalysis: Urine output, urine protein content, urine specific gravity, microalbuminuria, glycosuria, and the presence of injury markers (NGAL, KIM-1 etc).
- Blood Urea Nitrogen (BUN): Surrogate marker of waste removal.
- Electrolyte Balance: Assessing electrolyte levels (Na+, K+, Ca2+, Cl–) in blood and urine to determine renal electrolyte homeostasis.
- Metabolic State: The kidney plays a crucial role in acid-base homeostasis; assessing blood and urine pH and bicarbonate levels enables base excess/deficit to be calculated. Blood metabolic profile (glucose, lactate, bicarbonate), and both oxygen consumption and tissue oxygenation (to assess oxygen usage and levels in kidney tissue are used in combination to evaluate the mechanistic action and outcome of acidosis or alkalosis.
- Glucose and Amino Acid Handling: Assessing the reabsorption of glucose and amino acids by the renal tubules, which is crucial for metabolic homeostasis.
- Renal Haemodynamics: Renal blood flow, arterial perfusion pressure and intra-renal resistance are continually monitored in real-time.
- Renal Tubular Function Tests: Assessing the function of different segments of the renal tubules, such as the ability to reabsorb or secrete substances.
- Renin-Angiotensin-Aldosterone System (RAAS) Activity: Evaluating the components and activity of the RAAS, which plays a key role in regulating blood pressure and fluid balance.
- Blood Co-Oximetry: Haemoglobin saturation (total, deoxy, carboxy, met) and O2/CO2 (saturation and partial pressure/tension) are recorded.
- Drug Metabolism and Clearance Studies: Evaluating drug are metabolism and clearance within the kidney is essential for pharmacokinetic studies.
- Nephrotoxicity Assessment: Assessing the impact of drugs or toxins on kidney tissue, functional parameters (i.e. haemodynamics, gluconeogenesis, inflammatory reactions) using viability assays, biomarker analysis, and histopathology.
- Portal Vein & Hepatic Artery Haemodynamics: Continuous monitoring of blood flow rate and pressure. enables the calculation of intravascular resistance.
- Metabolic State: The liver plays a crucial role in acid-base homeostasis; assessing blood pH and bicarbonate levels enables base excess/deficit to be calculated. Blood metabolic profile (glucose, lactate, bicarbonate), and combined oxygen consumption and tissue oxygenation (to assess oxygen usage and levels in liver tissue) are used to evaluate the mechanism of action of acidosis or alkalosis. Gluconeogenesis is calculated based on lactate clearance and conversion to glucose and/or bicarbonate.
- Blood Lactate: Elevated lactate can indicate impaired liver function or inadequate oxygen delivery.
- Hepatocyte & Endothelial Cell Damage Markers: Detect alanine transaminase (ALT) and aspartate transaminase (AST) using spectrophotometric assays.
- Bilirubin Levels: Monitoring both total and direct bilirubin levels helps assess the liver’s ability to process and excrete bilirubin.
- Liver Enzymes (AST, ALT, ALP, GGT): Elevated enzyme levels can indicate liver cell damage or biliary obstruction.
- Albumin Synthesis: Albumin levels reflect the liver’s synthetic function and overall health.
- Prothrombin Time (PT)/International Normalized Ratio (INR): Coagulation tests assess the liver’s ability to produce clotting factors.
- Ammonia Clearance: The liver’s ability to detoxify ammonia is crucial; impaired clearance can indicate liver dysfunction.
- Urea Synthesis: Assessment of urea production can provide insights into the liver’s protein metabolism.
- Ketone Body Production: Measuring ketone bodies helps determine the liver’s metabolic state.
- Bile Production & Flow: Bile production and flow through the biliary system are crucial for digestion and excretion of waste products. Bile analysis for composition (pH, electrolytes, glucose, lactate).
- Cholesterol & Triglyceride Synthesis: The liver’s role in fat metabolism can be evaluated by monitoring blood lipids.
- Drug Metabolism & Biotransformation: The liver’s ability to metabolise drugs is crucial; assessing this can guide pharmacokinetic studies.
- Hepatotoxicity Assessment: Evaluating the impact of substances on liver tissue through viability assays, biomarker analysis, and histopathology.
- Glycogen Storage & Mobilisation: Assessing glycogen levels can provide insight into the liver’s energy storage and supply functions.
- Plasma Protein Synthesis: The liver synthesises various plasma proteins; their levels can reflect hepatic synthetic capacity.
- Spleen Haemodynamics: Splenic arterial blood flow, arterial pressure, and intra-splenic resistance.
- Splenomegaly Response: Alterations in spleen dimensions and weight are recorded to confirm initiation, maintenance, or reversal of splenomegaly.
- Red Blood Cell (RBC) Functional Tests: The spleen performs a cardinal role in RBC homeostasis, via removal of deformed or dying cells; therefore, acting as a reservoir capable of autotransfusion to regulate haematocrit. Assays available include RBC count, reticulocyte count, haematocrit, total haemoglobin, and haemoglobin state (oxyhaemaglobin, deoxyhaemaglobin, carboxyhaemaglobin, methaemaglobin). Blood O2 and CO2 saturation, bilirubin (as a marker of haemoglobin breakdown), and ferritin/iron (to evaluate iron storage and metabolism) are also monitored.
- Leukocyte Functional Tests: The spleen is a highly immunological organ, acting as a reservoir for leukocytes. Assays include simple WBC counts through to advanced flow cytometry, which facilitates full phenotyping and functional characterisation of leukocytes, and phagocytic activity of spleen resident macrophages.
- Platelet Functional Tests: Platelet count to confirm spleen-dependent platelet storage/extravasation, functional characterisation (via surface P-selectin, GP11b/IIIa activation, annexin binding etc), platelet aggregation assays.
- Spleen Specific Soluble Immune Components: Immunoglobulin Levels (IgG, IgA, IgM), complement system components (C3, C4), cytokine Levels.
- Blood Glucose Regulation: Monitoring blood glucose levels and the pancreatic secretion of insulin and glucagon secretion help evaluate the organ’s role in glucose homeostasis.
- Metabolic Function: The pancreas has both exocrine and endocrine functions; assessing blood levels of glucose, insulin, and c-peptide enables evaluation of the endocrine function evaluation, while measuring enzyme production (amylase, lipase) reflects exocrine function.
- Pancreatic Artery & Vein Haemodynamic: Continuous monitoring of blood flow rate and pressure in the pancreatic vasculature. Vascular resistance can then be calculated to assess pancreatic blood supply.
- Insulin and C-Peptide Secretion: Measurement of insulin and c-peptide provides insights into β-cell function and insulin synthesis.
- Somatostatin Secretion: Assessing somatostatin secretion can provide insights into δ-cell function and the regulation of pancreatic hormone release.
- Pancreatic Islet Cell Viability: Assessing the viability of islet cells using viability assays or histology.
- Glucagon Secretion: Measurement of glucagon helps assess α-cell function and the pancreas’s response to hypoglycaemia.
- Pancreatic Polypeptide (PP) Levels: Measuring PP can reflect the functional status of the F cells and their response to hypoglycemia.
- Electrolyte Composition of Pancreatic Secretions: Electrolyte analysis of pancreatic secretions (Na+, K+, Cl–, HCO3) can provide insights into the exocrine function and ductal cell activity.
- Histological Examination: Microscopic analysis of pancreatic tissue can reveal structural integrity, fibrosis, or other pathological states.
- Bicarbonate Secretion: Assessing bicarbonate levels in pancreatic juice helps evaluate the pancreas’s ability to neutralize stomach acid in the duodenum.
- Amylase & Lipase Levels: Elevated levels of these enzymes in the blood can indicate pancreatic damage or stress.
- Lactate Levels: Elevated lactate may indicate impaired pancreatic function or inadequate oxygen delivery to the tissue.
- Drug Metabolism & Biotransformation: Evaluating the pancreas’s ability to metabolize drugs, although less prominent than the liver, is still crucial for certain medications.
- Protein Synthesis: Measurement of protein levels secreted by the pancreas, including digestive enzymes and bicarbonate, to assess synthetic function.
- Inflammation Markers: Assessing cytokine and chemokine levels can provide insights into the inflammatory state of the pancreas.
- Enzyme Activation & Functionality: Quantify the activity of pancreatic enzymes like trypsin, chymotrypsin, carboxypeptidases, which are essential for digestion.
- Pancreatic Perfusion Efficiency: Evaluate the effectiveness of the ex-vivo perfusion in maintaining physiological levels of oxygen and nutrient delivery to the pancreatic tissue.
Preservation Protocols:
- Hypothermic Machine Perfusion: Assess the efficacy of cold perfusion systems in maintaining limb viability.
- Normothermic Machine Perfusion: Study the impact of warm perfusion on tissue metabolism and function.
- Preservation Solutions: Compare different preservation solutions and their effects on vascular endothelium and muscle tissue integrity.
- Cryopreservation Techniques: Experiment with freezing protocols to extend the limb’s viability window for transplantation.
Preservation Device & Solution Testing:
- Custom Device Evaluation: Design and test novel limb preservation devices, including perfusion systems, temperature control systems, oxygenators, and extracorporeal devices.
- Bioreactor Development: Use bioreactors to simulate physiological conditions and assess their impact on limb preservation and recovery.
- Preservation/Perfusion Solutions: Controlled evaluation of novel preservation and perfusion solutions.
Reperfusion Response:
- Metabolic Assessment: Measure metabolites such as lactate, pyruvate, and ATP to evaluate metabolic recovery post-reperfusion.
- Oxidative Stress Markers: Quantify reactive oxygen species (ROS) and antioxidant levels to assess oxidative damage and protective responses.
- Endothelial Function: Assess vascular response using endothelial-dependent and independent vasodilators and monitor for signs of reperfusion injury such as increased vascular permeability.
Skin Rejection Monitoring:
- Histopathology: Perform a detailed microscopic skin biopsy examination to identify the presence of infiltrating leukocytes, such as T lymphocytes and macrophages. Identify specific pathological features such as interface dermatitis, spongiosis, and necrotic keratinocytes, which are indicative of acute skin rejection.
- Immunofluorescence: Use direct immunofluorescence to detect deposition of immunoglobulins, complement components, and fibrinogen at the dermoepidermal junction to confirm antibody-mediated rejection.
- Molecular Techniques: qPCR/microarrays to quantify gene expression profiles associated with rejection, including upregulation of cytokines, chemokines, and adhesion molecules. Quantify donor-specific antibodies (DSA) that mediate rejection through complement-dependent cytotoxicity or antibody-dependent cellular cytotoxicity.
- Dermoscopy: Non-invasively observe changes in skin pigmentation and vascular patterns of the skin that are indicative of rejection.
Muscle Rejection Monitoring:
- Electromyography (EMG) & Nerve Conduction Studies: Perform EMG to detect abnormal electrical activity in muscles that indicate inflammation due to rejection. Nerve conduction studies to reveal decreased conduction velocity or amplitude associated with immune-mediated nerve damage.
- Muscle Biopsy with Enzyme Histochemistry: Assess the activity of various muscle tissue enzymes, such as myophosphorylase or acid phosphatase, which can be altered in the presence of rejection.
- Immunohistochemistry (IHC): Detect specific markers associated with muscle injury and regeneration, e.g. major histocompatibility complex (MHC) class I overexpression, which is a sign of immune activation.
Vascular Rejection Monitoring:
- Endothelial Cell Activation Markers: Quantify blood-soluble markers of endothelial activation in blood, such as Von Willebrand factor (VWF), soluble intercellular adhesion molecule-1 (sICAM-1), and soluble vascular cell adhesion molecule-1 (sVCAM-1), which indicate endothelial injury and potential rejection.
- Vascular Imaging: Use ultrasound to detect vascular abnormalities such as stenosis, thrombosis, or vessel wall irregularities that may be indicative of rejection.
- Arterial Biopsy: Histological examination of arterial biopsies to reveal features of vasculitis, intimal thickening, or fibrinoid necrosis characteristic of vascular rejection.
- Gene Expression Profiling: Analyse gene expression in vascular tissues for signs of rejection, focusing on genes related to immune response, endothelial injury, and smooth muscle cell activation.
Clinically Relevant Diagnosis:
- Banff Classification: The Banff classification system provides standardised diagnostic criteria for acute rejection in transplanted organs, including skin, muscle, and vessels. The system is based on histological examination of biopsy samples and includes criteria for the grading of rejection severity.
- Biomarker Panels: Including a combination of soluble molecules, cell-free DNA, microRNAs, and/or other circulating factors specific to rejection.
- Monitor clinical signs of allograft dysfunction, including changes in the appearance or function of the transplanted limb.
Drug Delivery Studies:
- Absorption Studies: Measure drug tissue penetration via intravenous, intra-arterial, or topical routes using pharmacokinetic modelling.
- Sub-dermal Drug Delivery: Test the effectiveness of subcutaneous injections or implantable drug delivery systems.
- Topical Immunomodulation: Apply immunosuppressive agents directly to the skin and monitor local and systemic absorption and effects.
Immunosuppressant Delivery:
- Intravenous Administration: Study pharmacodynamics and pharmacokinetics of systemic immunosuppressant drugs.
- Topical Application: Assess the potential for local immunosuppression without systemic side effects by applying drugs to the skin or muscle.
- Drug Efficacy: Monitor leukocyte populations and rejection markers to determine the effectiveness of various immunosuppressive regimens.
Functional Assessment:
- Muscle Strength & Fatigue: Evaluate post-transplant muscle contractility and endurance using electromyography and force measurement systems.
- Nerve Function: Monitor nerve function and recovery through electrophysiological testing and electrode implantation assessment.
- Vascular Assessment: Use Doppler ultrasound and angiography to assess blood flow dynamics and ensure vascular latency.
Skin Evaluation:
- Histological & Immunohistochemical Analysis: Assess skin biopsies for cellular structure, density, and distribution of various cell types. Evaluate the expression of specific proteins such as collagen, elastin, and keratins.
- Confocal Microscopy: Real-time non-invasive imaging to visualize the skin layers, cell morphology, and blood flow.
- Transepidermal Water Loss (TEWL): Measure water evaporation volume through the skin to assess barrier function.
- Cutometry & Elastometry: Quantify the mechanical properties of skin, such as elasticity and firmness.
- Photographic Assessment: Document skin colour, texture, and any visible changes over time.
Muscle Evaluation:
- Muscle Biopsy with Histopathology: Analyse muscle fibre type, size, distribution, and presence of any pathological changes.
- Electromyography (EMG): Measure skeletal muscle electrical activity and neuromuscular function.
- Muscle Function Tests: Evaluate strength, endurance, and fatigue resistance through dynamometry and functional performance tests.
- Myofibrillar ATPase Staining: Assess muscle fibres through differentiation of slow and fast-twitch muscle composition.
Fascia Evaluation:
- Ultrasound Elastography: Measure the elasticity and mechanical properties of fascial tissue in response to stress.
- Tensiometry: Test the tensile strength and viscoelastic properties of fascial samples.
- Histological Staining: Visualize the arrangement and density of collagen fibres and assess pathological changes within the fascia.
- Immunohistochemistry (IHC): Identify and localize specific proteins or markers within the fascia, such as fibronectin or tenascin-C.
Nerve Evaluation:
- Electrophysiological Studies: Perform nerve conduction studies (NCS) and electromyography (EMG) to assess nerve function and integrity.
- Nerve Biopsy: Examine the ultrastructure of nerve fibres, myelin, and Schwann cells.
- Immunofluorescence & Immunohistochemistry: Detect specific markers of nerve regeneration and damage, such as neurofilaments and S100 proteins.
- Functional Assessment: Conduct sensory and motor function tests, such as two-point discrimination, Semmes-Weinstein monofilament testing, and reflex testing.
Bone & Tendon Evaluation:
- Bone Evaluation: Determine bone strength and fracture resistance with compression, bending and torsion tests.
- Ultrasound Imaging & Elastography: Visualize tendon structure and assess mechanical properties such as strain and stiffness.
- Biomechanical Testing: Measure tensile strength, elasticity, and fatigue resistance using mechanical testing devices.
- Histological Analysis: Evaluate tendon structure, collagen fibre orientation, and cellularity.
- Immunohistochemistry (IHC): Detect specific markers of tendon healing and regeneration, such as scleraxis or tenomodulin.
Cartilage Evaluation:
- Arthroscopy: Direct visualization of cartilage surface for signs of wear, tears, or defects.
- Biochemical Assays: Analyse cartilage samples for content of glycosaminoglycans, collagen, and other extracellular matrix components.
- Mechanical Indentation Testing: Determine the compressive properties and resilience of cartilage tissue.
Evaluating Lymphatic Vessels & Vasculature:
- Lymphangiography: Use imaging to assess the integrity and function of lymphatic drainage in the transplanted limb.
- Immunofluorescence: Label lymphatic endothelial markers like LYVE-1 or podoplanin to visualize lymphatic networks.
- Endothelial Function Testing: Use flow-mediated dilation or acetylcholine challenge to assess the health of the vascular endothelium.
Recipient Leukocyte Infiltration:
- Immunohistochemistry & Immunofluorescence: Label and quantify infiltrating recipient leukocytes.
- Flow Cytometry: Characterize the phenotypes and activation states of leukocytes from the recipient.
Cytokine Response:
- Multiplex Assays (e.g. Luminex, Cytometric Bead Array): Measure the levels of multiple cytokines simultaneously in the serum, perfusate, or tissue lysates.
- ELISA: Quantify individual cytokines to assess the inflammatory and immune response.
Characterising Donor Resident Leukocytes:
- Langerhans Cells: Use Langerin and CD1a staining to identify resident skin leukocytes.
- Donor CD4+ T Cells: Label with CD4 antibodies and use flow cytometry and/or immunohistochemistry.
- Dendritic Cells & Macrophages: Via CD11C, CD68, or CD163
Graft Infiltration & Graft-versus-Host Disease (GVHD):
- Biopsy & Histological Examination: Look for signs of GVHD such as apoptosis, lymphocytic infiltration, and tissue damage.
- Chimerism Studies: Use fluorescent in situ hybridization (FISH) or PCR for Y chromosomes (to identify male donor to female recipient or female donor to male recipient), or polymorphic markers to differentiate between donor and recipient cells.
Surgical Development:
- Technique Refinement: Use the model to practice and perfect surgical approaches to transplantation, anastomosis techniques, and nerve repair procedures.
- Microsurgery Skills Training: Employ the model for surgeons to enhance skills in microvascular and nerve suturing under a microscope.
- Reconstructive Surgery Protocols: Develop and test new reconstructive strategies to improve functional outcomes.
- Myocardial Energy Metabolism: Analyse substrates including lactate and pyruvate in perfusate via ABG.
- Haemodynamic Monitoring: Employ echocardiography for functional assessments, and record arterial pressure, flow and resistance.
- Assessing Perfusate for Inflammatory Markers: Measure cytokines using ELISA.
- Functional Assessment: Utilize scintigraphy for ventilation/perfusion scanning.
- Monitoring for Signs of Acute Lung Injury: Observe for oedema and haemorrhage in histological samples.
Viability Assessments
- Measure cellular energy via luciferase-based bioluminescence assays.
- MTT Assay to evaluate cell proliferation
- LDH Release and trypan blue exclusion to assess cell membrane integrity
- Calcein AM/Propidium Iodide Staining to differentiate live/dead cells
- Malondialdehyde (MDA) to quantify lipid peroxidation
- Protein Carbonyls as a marker of oxidative protein damage
- Glutathione, 8-hydroxy-2-deoxyguanosine and ROS production to assess antioxidant status
- ROS Production
- Nitric Oxide Levels as a marker of vascular tone
- Oxygen Consumption Rate
- Krebs Cycle Enzyme Activities
- NAD+/NADH Ratio
- Ammonia Production
- Glucose and Lactate Concentration
- Bicarbonate Recycling via Cori-cycle
- Leukocyte Phenotyping: Flow cytometry with specific antibodies for leukocytes.
- Cytokines: Perform multiplex bead-based Luminex assays (33 cytokines available)
- Complement Activation Products: Measure C3a, C4a, C5a and MAC using immunoassays.
- Prostaglandin E2 (PGE2) & C-reactive protein (CRP): Marker of inflammation
- Endothelial Adhesion Molecules: Detect ICAM-1, VCAM-1 using immunohistochemistry or ELISA.
- Caspase-3 Activation: Marker of apoptosis
- TUNEL Assay: Detects apoptosis in tissue sections.
- Tissue Staining (e.g. Haematoxylin and Eosin): For general tissue architecture and cellular detail.
- Electron Microscopy: Provides ultrastructural details of cell and tissue damage.
- Fluorescence Microscopy: Visualizes specific molecules or cells tagged with fluorescent markers.
- Immunohistochemistry (IHC): Localised evaluation of markers of stress and damage.
- AI-Assisted Quantification: Utilizes artificial intelligence for more accurate and efficient analysis of IHC results.
- Ultrasound Imaging: Assesses blood flow in vascularized organs.
- Near-Infrared Spectroscopy (NIRS): Non-invasively measures tissue oxygenation.
- Standard Grading Scales (e.g. Banff Criteria & REMUZZI for Kidney Transplant): Assess organ transplant rejection and damage.
- Veterinary Pathologist Reports: Using established grading scores for comparison.
Molecular Biology
- Whole Exome Sequencing: Sequence all coding regions of the genome to identify genetic variations that may influence transplant outcomes.
- Transcriptome Profiling: Analyse the complete set of RNA transcripts to understand gene expression changes due to transplantation.
- Differential Protein Expression Analysis: Compare protein levels between samples using techniques such as 2D-DIGE (Two-Dimensional Difference Gel Electrophoresis) or mass spectrometry.
- Post-Translational Modification Analysis: Identify chemical modifications of proteins that could affect their function, using targeted mass spectrometry.
- Protein Biomarkers of Reperfusion Injury: Isolate and identify proteins associated with ischemia-reperfusion damage.
- Enzyme Activity Assays: Measure the activity of enzymes involved in various metabolic pathways affected by reperfusion.
- Immune Protein Analysis: Study the abundance and function of proteins involved in the immune response to the transplanted organ.
- Cytokine & Chemokine Levels: Quantify inflammatory mediators to evaluate the immune response post-transplantation.
- Mass Spectrometry: Utilise this technology to characterize proteins altered by reperfusion, analyse changes in protein expression, and investigate signalling pathways.
- Metabolic Profiling: Analyse the complete set of metabolites present in a biological sample to gain insights into metabolic alterations due to transplantation.
- Biomarker Discovery: Identify metabolites that serve as indicators of organ health or injury.
- Metabolite Alterations: Measure the levels of specific metabolites that reflect reperfusion injury.
- Organ Function Metabolic Markers: Monitor metabolites related to the function of the specific organ transplanted.
- Energy Metabolism Assessment: Evaluate the production of ATP and the overall energy status of the organ.
- Drug Metabolism & Toxicity: Assess the effects of immunosuppressive drugs on organ metabolism and potential toxicity.
- Lipid Profile Alterations: Analyse changes in the lipid composition that may result from reperfusion injury.
- Membrane Lipid Integrity: Evaluate the stability and integrity of cellular membranes by assessing the composition of membrane lipids.
- Gene Expression Changes: Use RNA sequencing or microarrays to identify alterations in gene expression patterns due to reperfusion.
- Gene Expression Analysis in Donor/Recipient Leukocytes: Investigate the impact of transplantation on leukocyte gene expression and identify genes linked to immune response and graft rejection.
- Non-coding RNA Profiling: Examine the roles of microRNAs and other non-coding RNAs in the regulation of genes related to reperfusion injury.
- Immune Response Gene Profiling: Assess gene expression patterns related to the immune response to the transplanted organ.
- MHC Typing & Matching: Determine the compatibility between donor and recipient major histocompatibility complex (MHC) alleles.
- ABO Incompatible Transplantation: Explore the effects of ABO blood group incompatibility on transplant outcomes.
- Male/Female Mismatched Transplantation: Track the movement of donor or recipient leukocytes using male chromosome markers, providing insight into leukocyte migration and engraftment.
- Gene Expression Profiles Across Thousands of Genes: Use to assess the global impact of preservation methods on gene expression.
- RNA Interference (RNAi): Apply gene silencing techniques to investigate potential therapeutic targets for enhancing organ preservation.
- Gene Expression Profiling via RNA-Seq: Utilize next-generation sequencing to analyse gene expression changes during reperfusion and identify genes involved in ischaemia-reperfusion injury.
This is not an exclusive list of the services we offer. Our team of enthusiastic perfusionists are keen to push the boundaries of scientific research. If you have a model in mind, no matter how complex, please get in touch.
Pebble's Customisable
Service Package
Case Studies
Case Study 1: Evaluation of nanoparticle treatment of the donor kidney
A reactive oxygen species (ROS)-scavenging nanoparticle treatment aiming to reduce IRI was tested on an isolated LIVING-KIDNEY system. Kidneys were perfused with or without the nanoparticles for 6 hours. Following perfusion, both systems were reperfused with allogeneic blood, mimicking a transplant scenario.
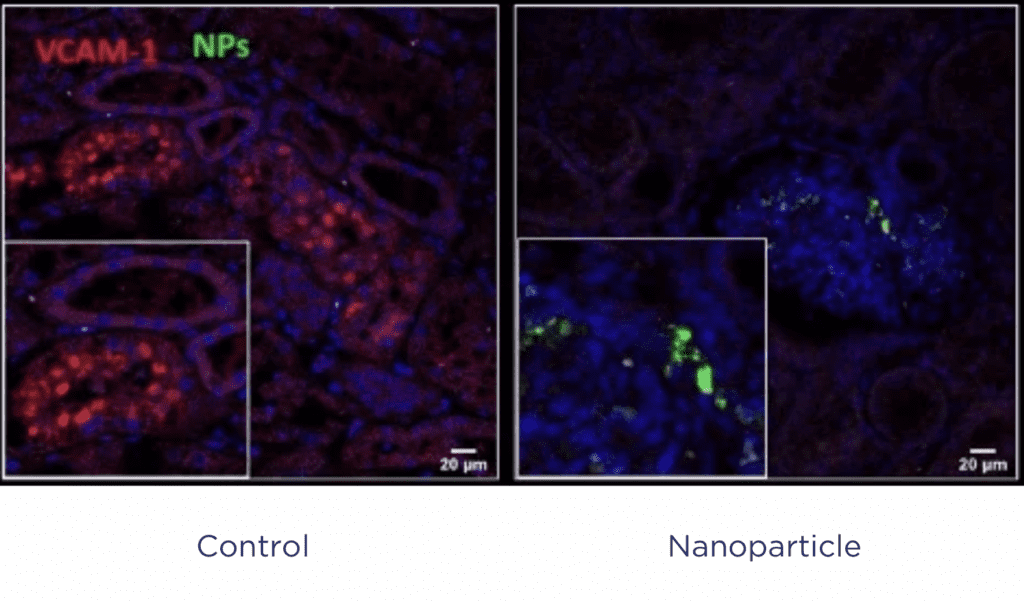
Treated kidneys showed improved haemodynamics with reduced tissue necrosis, indicating that the nanoparticle treatment not only successfully targeted the kidney, it also successfully reduced IRI in a translatable model.
Case Study 2: Developing an ABMR model for FDA submission
Pebble were asked to generate a model of antibody mediated rejection (ABMR) of the kidney to evaluate a novel therapy. The team phenotyped animals immediately prior to initiating the study, and established an ABO incompatible model, via an A type donor into an O type recipient.
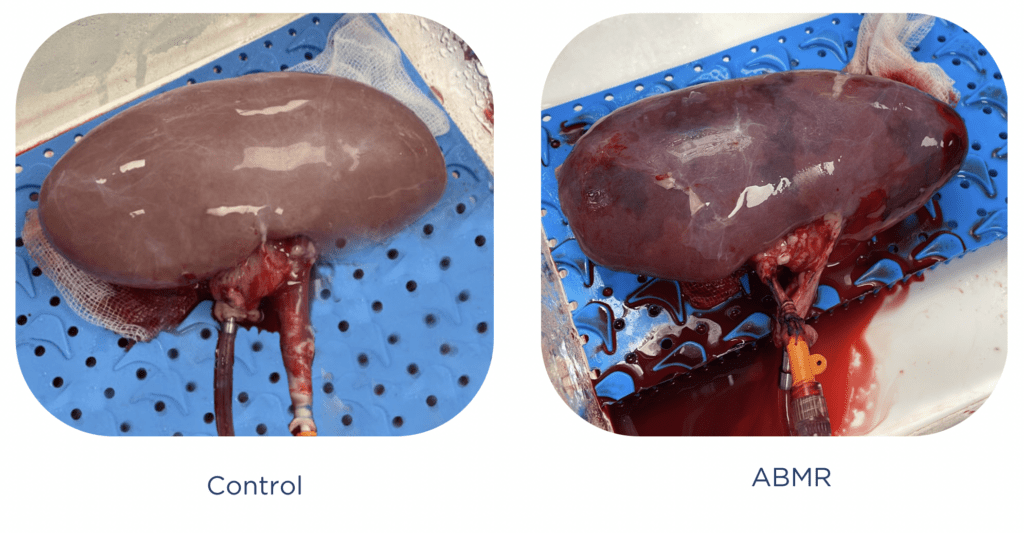
Pebble then performed a series of safety and efficacy evaluations assessing the novel therapy compared to gold standard treatment. Complex monitoring of organ function, gluconeogenesis, haemodynamic compromise, plasma and urinalysis, glomerular filtration rate, NGAL secretion, and detailed imaging of complement and tissue integrity were performed to confirm ABMR.
This composite data was then used to determine improvements in disease onset and severity via administration of the new asset. Pebble are now supporting the generation of a submission pack to the FDA to initiate a phase II clinical trial.
Why Choose Pebble?
Testimonials

Prof Richard D’Arcy
Department of Biomedical Engineering
Vanderbilt University, Nashville, USA
“We used Pebble to perform an early preclinical evaluation of novel polysulphide nanoparticle formulation with potent antioxidant properties. Pebble designed a series of experiments to replicate transplant reperfusion, and generated a clinically relevant datapack within a matter of weeks, demonstrating that our NPs were both safe and effective. We then requested additional studies to delineate optimal dosing and mechanism of action. Pebble further developed a bespoke kidney platform and confirmed our NPs were scavenging ROS and reducing reperfusion-associated inflammation
What impressed me the most was the efficiency of Pebble. They took care of experimental designs, performed all surgical procedures independently, collected and analysed data, and helped us prepare a manuscript under review in a high impact journal. This preclinical work is directly supporting our next phase of research, including an ambitious investment plan. It’s remarkable that the entire study was completed at a fraction of the cost of a large animal model, with a more streamlined regulatory process fully handled by Pebble. We will definitely use Pebble again in future endeavours.”

