Liver Studies
A Novel, Translatable Platform for Hepatic Research
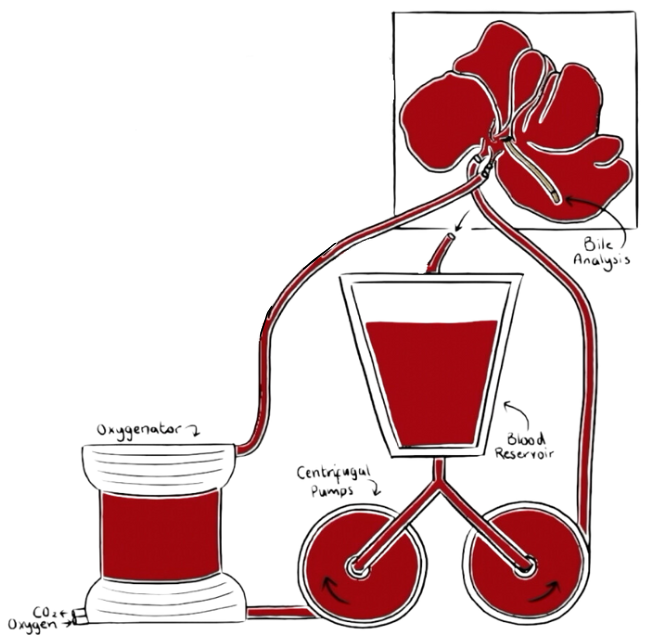
Pebble's LIVING-LIVER System
The Pebble LIVING-LIVER system replicates in-vivo physiology by connecting it to a clinical life-support system supplying the vasculature with a physiological blood supply. Warm, nutrient-rich, oxygenated blood is circulated through the hepatic artery, and deoxygenated, nutrient-depleted blood through the portal vein. Haemodynamics can be independently controlled within the vasculature, providing physiological pressures and blood flow.
Liver function and biochemistry are restored, enabling biological processes such as gluconeogenesis, glycolysis, glycogenesis, protein production (including clotting factors), bile and enzyme production.
We provide an advanced platform to accurately evaluate Drug-Induced Liver Injury (DILI), crucial for drug development and safety assessments. Identifying early indicators of potential hepatotoxicity enables the rapid modifications of drug candidates, thereby enhancing safety and efficacy.
Hepatic Evaluation Services
LIVING-LIVER System Models
- Testing the efficacy of immunomodulatory therapies in reducing transplant rejection.
- Monitoring biomarkers associated with graft health and rejection.
- Assessing new surgical techniques and preservation solutions for hepatic transplantation.
- Assess preservation techniques and solutions for storing livers prior to transplantation.
- This includes evaluating the efficacy of different preservation solutions, devices, temperatures and additives in maintaining liver viability and function.
- The LIVING-LIVER system provides a physiologically relevant platform for evaluating the efficacy and toxicity of drugs on the liver.
- We can assess their effects on liver function and metabolism, aiding drug development and safety assessment.
- Biodistribution, pharmacokinetics and pharmacodynamics can be determined.
- Tropism, dosing and toxicity can also be determined.
- The LIVING-LIVER system can be used to study immune activation and regulation, as well as the role of the liver in immune related diseases and therapies.
- We can induce immune-mediated injury, mimicking auto-immune liver diseases.
- We can simulate cessation, or reduction, of blood flow followed by its restoration to study mechanisms of damage and repair.
- We can therefore evaluate therapeutic agents designed to mitigate ischaemia-reperfusion injuries.
- We can model toxic liver injury by perfusing the liver with hepatotoxic substances, mimicking drug-induced liver injury observed in clinical settings.
- We can induce hypoxic injury by altering blood oxygen levels.
- By reducing oxygen supply or blood flow rates, we can create hypoxic conditions leading to cellular injury, oxidative stress and inflammation.
- This model can mimic conditions such as hypoxic hepatitis observed in patients with severe hypotension or cardiac arrest.
- We can model biliary injury by perfusing the liver with bile acids or cholestatic agents.
- This induces bile duct injury, cholestasis and liver damage, mimicking conditions such as primary biliary cholangitis or obstructive cholestasis.

Liver Specific Testing
- Blood flow rate and pressure can be continually monitored.
- Intra-vascular resistance can then be calculated as an indicator of vascular tone.
- Bile Production & Composition: Bile production and flow through the biliary system are crucial for digestion and excretion of waste products. Bile composition can be analysed to provide insights into liver function.
- Glucose Production & Glycogen Storage: The liver plays a key role in the body’s energy supply. Tests can evaluate its efficiency in glucose production (gluconeogenesis) and storage as glycogen.
- Plasma Protein Synthesis: The liver synthesises various plasma proteins; their levels can reflect hepatic synthetic capacity.
- Bilirubin Levels: We can monitor both total and direct bilirubin levels helps assess the liver’s ability to process and excrete bilirubin. High levels can also act as an indicator of liver dysfunction.
- Albumin Synthesis: Albumin levels can be measured as an indicator of the liver’s synthetic function and overall health.
- Ketone Body Production: The liver produces ketone bodies in a process called ketogenesis, providing an alternative energy source during periods where glucose is scarce. Changes in ketone production and metabolism can be indicators of liver health and function.
- Ammonia Conversion: The conversion of ammonia to urea in the liver is crucial. Impaired conversion of ammonia can indicate liver dysfunction, therefore we can assess both urea and ammonia concentrations as a marker of liver health and function.
- Blood Clotting Factors: The liver produces proteins that are crucial for blood clotting. Prothrombin time (PT) and partial thromboplastin time (PTT) tests can assess the liver’s ability to produce these proteins.
- A metabolic profile of the blood, including blood pH and bicarbonate levels, can be determined to assess acidosis or alkalosis.
- Gluconeogenesis is calculated based on lactate clearance and conversion to glucose and/or bicarbonate.
- Tissue oxygenation and oxygen consumption can be measured to assess oxygen usage and levels in liver tissue.
- Blood lactate concentrations can be continually monitored. Elevated lactate can indicate impaired liver function or inadequate oxygen delivery.
- We can measure levels of enzymes, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT). Elevated levels can indicate liver cell damage or biliary obstruction.
- The liver’s role in lipid metabolism can be evaluated by monitoring blood lipids.
- Analysis of the types and concentrations of fatty acids in the liver can indicate metabolic status, particularly related to fatty liver disease or metabolism.
- The liver’s ability to metabolise drugs is crucial; assessing this can guide pharmacokinetic studies.
- We can evaluate the impact of substances on liver health through viability assays, biomarker analysis, and histopathology.
Therapeutic Delivery
- Direct intravenous injection allows drugs to enter the circulation and reach the liver.
- Hepatic artery infusion involves delivering drugs directly into the hepatic artery, which supplies oxygenated blood to the liver.
- This method ensures a high concentration of the drug is delivered to the liver while minimizing exposure to other tissues.
- Portal vein infusion can be carried out to assess targeted delivery of substances to the liver.
- For localized treatment, drugs can be injected directly into the liver tissue.
Imaging Analysis
- Can be used to determine blood flow and soft tissue imaging.
- Ultrasound can also be used for localised delivery of therapeutics.
- Monitor temperature distribution across the entirety of the organ.
- Temperature differentials can be determined, indicating any potential regions of ischaemia within the perfused organ, or any negative impact of the tested therapy.
Molecular Biology
- Targeted Gene Sequencing: Focused sequencing of specific genes or regions to confirm the integration of therapeutic cargos.
- Transcriptome Profiling (RNA-Seq): Detailed analysis of the transcriptome to evaluate gene changes induced by therapies.
- Epigenetic Analysis: Assessment of DNA methylation and histone modifications to understand epigenetic changes influenced by therapies, which can impact gene expression and cellular behaviour.
- Gene Expression Profiling: Quantitative analysis of gene expression patterns pre- and post-therapy, providing insights into therapeutic efficacy and biological impact.
- Differential Protein Expression Analysis: Compare protein levels between samples using techniques such as two-dimensional difference gel electrophoresis or mass spectrometry.
- Post-Translational Modification Analysis: Identify chemical modifications of proteins that could affect their function, using targeted mass spectrometry.
- Protein Biomarkers of Injury: Isolate and identify proteins associated with injury following therapeutic administration.
- Enzyme Activity Assays: Measure the activity of enzymes involved in various metabolic pathways affected by therapeutic delivery/exposure.
- Immune Protein Analysis: Study the abundance and function of proteins involved in the immune response to therapeutics.
- Cytokine & Chemokine Levels: Quantify inflammatory mediators to evaluate the immune response to therapeutic substances.
- Mass Spectrometry: Characterize proteins altered by therapeutic administration, analyse changes in protein expression, and investigate signalling pathways.
- Metabolic Profiling: Analyse the complete set of metabolites present in a biological sample to gain insights into metabolic alterations due to the therapy, or response to the therapy.
- Biomarker Discovery: Identify metabolites that serve as indicators of organ health or injury.
- Metabolite Alterations: Measure the levels of specific metabolites that reflect the efficacy of the therapy, or response to the therapy.
- Organ Function Metabolic Markers: Monitor metabolites related to the function of the specific organ under treatment.
- Energy Metabolism Assessment: Evaluate the production of ATP and the overall energy status of the organ.
- Drug Metabolism & Toxicity: Assess the effects of therapeutics on organ metabolism and potential toxicity.
- Lipid Profile Alterations: Analyse changes in the lipid composition that may result from the efficacy of the therapy, or response to the therapy
- Membrane Lipid Integrity: Evaluate the stability and integrity of cellular membranes by assessing the composition of membrane lipids.
- Gene Expression Changes: Use RNA sequencing or microarrays to identify alterations in gene expression patterns due to exposure to therapeutics.
- Non-coding RNA Profiling: Examine the roles of microRNAs and other non-coding RNAs in the regulation of genes related to treatment.
- Immune Response Gene Profiling: Assess gene expression patterns related to the immune response to the therapy.
- Nucleic Acid Microarrays: Profiles gene expression across thousands of genes: Use to assess the global impact of treatment, or the response to treatment on gene expression.
- Gene Expression Profiling via RNA-Seq: Utilize next-generation sequencing to analyse gene expression changes during treatment.

Histopathological Assessment
- Provides a general overview of tissue architecture, highlighting nuclei in blue-purple (haematoxylin) and cytoplasm in pink (eosin).
- Useful for assessing overall liver morphology and detecting pathological changes.
- Different trichrome stains, such as Masson’s trichrome, can highlight collagen fibres in blue and help evaluate the extent of fibrosis or scarring in the liver.
- Stains glycogen and certain pathological deposits in magenta, useful for assessing changes in glycogen storage or detecting specific liver pathologies.
- Detects lipid droplets, particularly useful for assessing the presence and accumulation of fat in liver cells, which is relevant in conditions like non-alcoholic fatty liver disease (NAFLD).
- Used to visualise reticulin fibres, aiding in the assessment of liver cell architecture and potential changes in the hepatic reticulin framework.
- Utilises specific antibodies to detect and visualize the expression of proteins or markers of interest, such as inflammatory markers, apoptosis-related proteins, or drug metabolizing enzymes in liver cells.
- TUNEL (Terminal deoxynucleotidyl Transferase dUTP Nick End Labelling): Used to identify apoptotic cells by labelling the free 3′-OH DNA ends, helping assess the extent of drug-induced apoptosis in the liver.
- Cleaved Caspase-3 Staining: Detects the presence of activated caspase-3, a marker of apoptosis, providing information about drug-induced cell death in the liver.
- Bromodeoxyuridine (BrdU): Used to assess cell proliferation by detecting the incorporation of BrdU into newly synthesized DNA, providing information on the regenerative capacity of liver cells.
- Glutamine Synthetase Staining: Highlights the expression of glutamine synthetase, an enzyme involved in ammonia detoxification, which can be relevant in studies involving drug-induced liver injury.
- We will provide a full histopathological report compiled by a blinded histopathologist.
- We are able to provide both qualitative and quantitative reports, alongside fixing and processing tissue samples.
We offer a streamlined service to evaluate your innovations. Our platforms enable fast-tracking of innovations towards FDA submission and subsequently into clinics. For more information, please don’t hesitate to contact one of our scientists.
Pebble's Customisable Service Package