Cell and Gene Therapies
A Novel Platform to Rapidly Assess Your Therapies
The Cell & Gene Research Toolbox Needs Changing
The scientific R&D community must translate discoveries into tangible therapies that impact health in a meaningful way. This is a challenging task, obstructed by laboratory models that have not changed in decades…
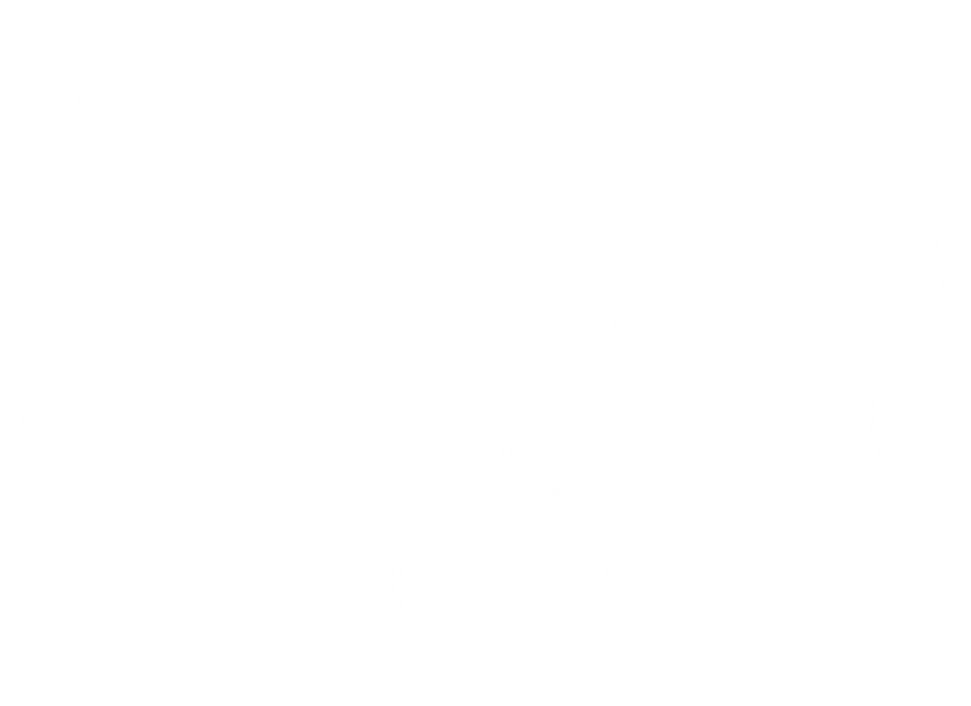
Pebble's LIVING-ORGAN Systems
The Pebble LIVING-ORGAN systems utilise porcine organs surplus to the food industry, eliminating the need for laboratory animals in our research. We are able to replicate in-vivo physiology, circulating warm, nutrient rich, oxygenated blood through arteries and deoxygenated, nutrient depleted blood through veins. This restores tissue homeostasis and organ function.
We offer bespoke systems and protocols in the following models:
What We Offer
Pebble’s LIVING-ORGAN systems provides in-depth analysis for the development and safety assessment of advanced therapeutics, including cell, gene, exosome and nanoparticle therapies.
This innovative platform simulates human physiology, enabling detailed immunogenicity, toxicity, biodistribution, metabolism studies and viral transduction assessments. By facilitating the evaluation of immediate immune responses and offering pharmacokinetic and pharmacodynamic profiling, the LIVING-ORGAN system guides the development of safer and more effective treatments for clinical use.
Additionally, it supports the refinement of organ-targeted delivery and personalised dosing strategies, essential for precision medicine. The LIVING-ORGAN system ensures that new therapies are primed for success from the laboratory to the patient.
Our multi-organ LIVING-ORGAN system provides a unique platform for mapping the biodistribution and tropism of cell, gene, exosome and nanoparticle therapies. By administering therapies into a central blood reservoir, we can trace their journey through an interconnected organ network, uncovering tropisms for specific organs or cells and revealing comprehensive biodistribution profiles.
We have significant experience in the evaluation of cell and gene therapies, including but not limited to:
Gene Therapies
Stem & Adipose-Derived Cells
Antibodies
Cell Therapies

Exosomes
Therapeutic Delivery Systems
Viral & Cell Vectors
AAV & CMV Nanomedicines
Perfusion Protocols
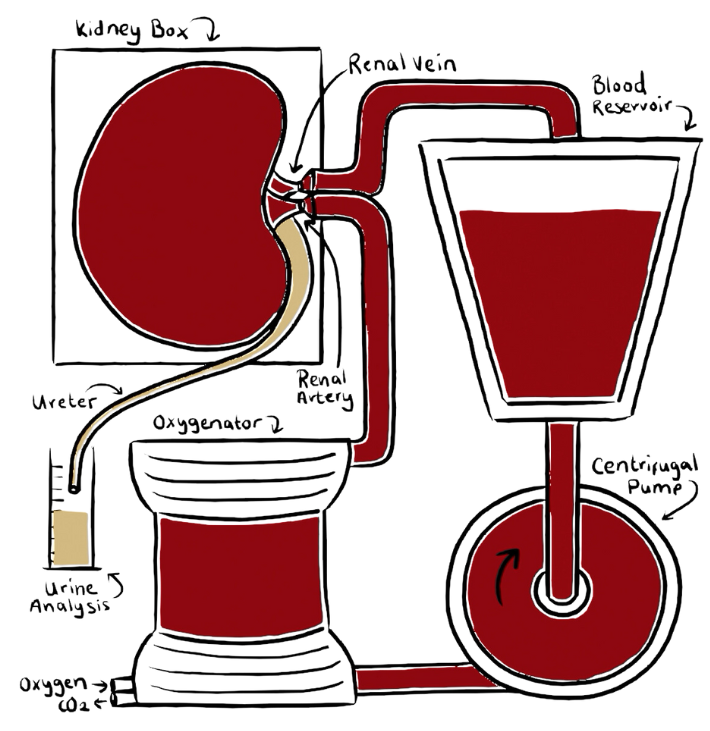
Organs are perfused with autologous blood and maintained under physiological conditions.
Our LIVING-ORGAN system reproduces the living environment. We replicate aortic and IVC blood flow, with oxygenated arterial blood and deoxygenated venous blood.
We only use clinical gold standard protocols for organ preservation (including approved cold-storage and machine perfusion approaches). All solutions, consumables and hardware are FDA/MHRA/EMA approved.
Pebble have performed perfusion studies for over 20 years, with more than 1,200 procedures to date – our expert multidisciplinary team are world-leading in this area.
Pebble’s LIVING-ORGAN systems are complex, for more information watch this video:
Therapeutic Evaluation Services
LIVING-ORGAN Systems for Therapy Testing
- Renoprotective Effects: Study cell-based therapies for their potential to facilitate repair and regeneration in kidney tissues.
- Gene Silencing/Overexpression: Evaluate the efficacy of gene therapies in modulating genes implicated in renal diseases, using advanced molecular analysis.
- Delivery to Renal Cells: Examine the ability of nanoparticles and exosomes to effectively deliver therapeutic agents to specific renal cell types.
- Functional Restoration: Assess therapies aimed at enhancing renal function, including glomerular filtration and tubular reabsorption.
- Kidney Function Evaluation: Our tests include measuring serum creatinine and blood urea nitrogen (BUN), as well as performing urinalysis for biomarkers such as NGAL and KIM-1, to assess renal function and nephrotoxicity
- Targeted Delivery and Expression: Evaluate the efficiency of cell and gene therapies in targeting liver cells, assessing transfection/transduction efficiency and gene expression.
- Therapeutic Efficacy: Study the impact of therapies on liver-specific functions like protein synthesis, detoxification, and metabolic regulation.
- Safety and Toxicity: Monitor liver enzyme levels and histopathological changes to assess potential toxicity and off-target effects.
- Liver Function Assessment: We measure serum levels of liver enzymes, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), as well as bilirubin and albumin, to monitor liver function and identify hepatotoxicity.
- Biodistribution and Uptake: Track the distribution and uptake of exosomes and nanoparticles within the liver using imaging and molecular techniques.
- Functional Impact: Assess the impact of these therapies on liver regeneration, fibrosis reduction, and immune modulation.
- Pharmacokinetics & Pharmacodynamics: Analyse the release profile of drugs or genetic material from nanoparticles and their subsequent effects on liver function.
- Immunomodulation Assessment: Evaluate the ability of therapies to modulate immune cell populations and cytokine production in the spleen.
- Gene Editing Efficacy: Test the precision and efficiency of gene editing tools in modifying splenic cells, particularly for haematological diseases.
- Leukocyte Targeting: Investigate targeting strategies for delivering therapies specifically to leukocytes within the spleen.
- Impact on Splenic Functions: Assess the effects of therapies on the spleen’s filtration capacity and its role in blood cell homeostasis.
- Islet Cell Regeneration: Evaluate therapies focused on replacing pancreatic islet cells.
- Gene Modulation in Pancreatic Disorders: Test gene therapies targeting specific pancreatic genes involved in diseases like pancreatitis.
- Targeted Delivery to Pancreatic Tissue: Investigate the efficiency of exosome and nanoparticle systems in delivering drugs or genetic material specifically to pancreatic tissues.
- Impact on Endocrine & Exocrine Functions: Measure the effect of therapies on insulin secretion, glucose metabolism, and digestive enzyme production.
- Pancreatic Function: We analyse serum amylase and lipase levels, as well as glucose tolerance, to detect pancreatotoxic effects of the therapies
- Integrated Organ Network: Our state-of-the-art perfusion system closely mimics the human body’s physiological conditions by integrating multiple organs — e.g. a liver, spleen, kidney, and pancreas — into a single circuit. This unique setup allows for the simulation of organ interactions and systemic responses, providing an unparalleled platform for cell and gene studies.
- Artificial Circulatory System: We recreate the circulatory pathways, including arteries and veins, ensuring that each organ receives blood flow akin to physiological conditions. This setup facilitates the study of complex interactions between different organs, making our platform ideal for multifaceted research and therapeutic testing.
- Modular Design: Our system is designed for flexibility, allowing for the addition or removal of organs based on specific research needs. This modularity enables tailored studies focusing on specific organ interactions or systemic responses.
- Integrated Organ Ex-Vivo Perfusion Toxicity Testing: We evaluate the relationships between the function and/or severity of injury of different organs on the same system.
- Blood flow: Precise control and monitoring of blood flow are crucial in replicating physiological conditions. Our system is equipped with advanced technology to continuously monitor and adjust hemodynamic parameters, ensuring an environment that closely mimics in vivo conditions.
- Flow Probes & Sensors: We employ state-of-the-art flow probes and arterial and venous pressure sensors to monitor blood flow dynamics. These tools provide real-time data on blood flow rates, pressures, and vascular resistance in each organ, enabling detailed analysis of hemodynamic responses.
- Customizable Flow Dynamics: The system allows for the adjustment of flow rates and pressures to simulate various physiological and pathological conditions. This feature is essential for studying the effects of different hemodynamic profiles on organ function and therapy outcomes.
- Real-Time Biochemical Monitoring: We continuously monitor key blood biochemistry parameters such as lactate, glucose, bicarbonate, base excess/deficit, pH, electrolytes, and anion gap. This continuous monitoring allows for the immediate assessment of the metabolic state of each organ and the system as a whole.
- Organ-Specific Function Tests: Our system enables the evaluation of individual organ functions through specific biomarkers and functional assays. For example, we assess renal function through glomerular filtration rate (GFR) measurement, urine analysis, and renal haemodynamics; liver function through enzyme levels, bilirubin, and synthesis of proteins; and pancreatic function through insulin secretion and enzyme production.
- Advanced Imaging and Histology: We utilise cutting-edge imaging techniques and histological analysis to visualize and assess structural and functional changes within each organ.
Immunogenicity Studies
- Mixed Lymphocyte Reactions (MLR): We can transfuse unrelated leukocytes into a whole blood circuit and induce an MLR. We analyse T-cell proliferation markers to predict possible rejection or graft-versus-host disease (GVHD) scenarios, tailoring cell therapies for improved biocompatibility.
- T-Cell Repertoire Analysis: We can analyse the T-cell receptor (TCR) repertoire diversity post-therapy exposure, identifying any expansion of specific T-cell clones indicative of an immune response. We can also map circulating and intra-organ T cell phenotypes via flow cytometry.
- ELISA: Using enzyme-linked immunosorbent assays, we can quantitatively measure antibodies against therapeutic agents, evaluating the potential for an adverse humoral response that could neutralize therapeutic effects.
- B-Cell Epitope Mapping: By identifying B-cell epitopes on therapeutic agents, we can understand and possibly mitigate the immunogenicity of therapies, paving the way for more refined therapeutic designs.
- Immunophenotyping of B-Cells: Flow cytometry enables us to characterize changes in B-cell populations, identifying shifts that could suggest therapy-induced humoral responses.
- Quantitative Multiplex Bead Arrays: We are able to simultaneously quantify a broad array of up to 33 different cytokines from a single sample. This robust assay enables the detection of a comprehensive range of cytokines, encompassing both pro-inflammatory and anti-inflammatory mediators, as well as growth factors and chemokines.
- Sensitivity and Specificity: Our platform’s high sensitivity allows for the detection of cytokines at very low concentrations, ensuring that even the subtlest changes in the immune environment can be captured. The specificity of the assay ensures that each cytokine is accurately measured without cross-reactivity, providing confidence in the data.
- Dynamic Range: The broad dynamic range of our multiplex assays accommodates high and low expressers of cytokines, giving us the flexibility to detect wide variations in cytokine levels that are characteristic of different inflammatory responses.
- Temporal Cytokine Kinetics: We perform time-course studies to track the kinetics of cytokine release over time, providing insights into the dynamics of the inflammatory response following therapy administration. This temporal data can be crucial in understanding the onset, progression, and resolution of immune activation.
- Cytokine Correlation Analysis: By correlating cytokine levels with clinical outcomes and other biomarkers, we can identify key cytokines that may serve as predictive indicators of therapeutic efficacy or potential adverse reactions.
- Immune Signature Development: Cytokine data contribute to the development of an immune signature that characterises the response to therapy, which can be used to tailor treatments and mitigate adverse immune effects.
- Cytokine Network Analysis: We use advanced computational tools to analyse the interplay and regulatory networks of cytokines, enhancing our understanding of the complex immune response elicited by the therapies.
Toxicity Studies
- LDH Release Assay: We can quantify lactate dehydrogenase (LDH) released into the blood-perfusate from damaged cells, providing a marker for cell membrane integrity and cytotoxicity.
- Tissue Biopsy and Analysis: We conduct detailed examinations of biopsied tissues using both traditional staining techniques and advanced imaging to identify morphological changes or damage at the cellular level. We create pathology reports prepared by veterinary pathologists, and use validated scoring systems to grade the tissue.
- Immunohistochemistry (IHC): IHC is employed to visualize the expression of specific proteins that may indicate cellular stress, injury, or death in response to therapeutic agents.

Biodistribution and Tropism Studies
- We can introduce fluorescently tagged therapies into our system’s central blood reservoir and monitor their passage through the interconnected organs, capturing real-time data on systemic distribution and organ-specific uptake.
- Analyse organs known for sequestration of cell and gene therapies, such as the liver, to understand the extent of their involvement in therapy capture and metabolism, which often leads to excretion or inactivation.
- Apply qPCR and in situ hybridization to detect and quantify the presence of gene or mRNA from therapies at a cellular level within each organ, providing insights into cellular-level tropism.
- By studying multiple organs concurrently, we can compare the tropism of therapies, identifying preferential targeting that could impact therapeutic efficacy or cause off-target effects.
- Our IHC techniques enable us to visualize the precise localization of cell and gene therapies within the organ tissues. By using antibodies specific to therapeutic agents or associated markers, we can observe the direct interactions between these agents and the cellular components of the organ.
- Integrate quantitative digital pathology to measure the intensity and distribution of IHC staining, offering detailed insights into the concentration and persistence of therapies within specific organ regions.
- Correlate biodistribution data with functional assays to assess the impact of the localised therapy on organ function, providing a link between the therapy’s location and its physiological effects.
Precision Delivery of
Advanced Therapeutics
- We test ligands or antibodies designed for organ-specific targeting, analysing their binding affinity and specificity to an organ or cell type. Our platform allows for direct measurement of targeted uptake and binding kinetics, facilitating the optimisation of ligand-engineered therapies.
- By administering therapies into our multi-organ system, we can refine dosage and delivery techniques to enhance the concentration of therapeutic agents in the target organs. This optimisation is crucial for maximizing therapeutic effects and reducing potential off-target interactions.
- Pharmacokinetic Profiling: We measure the concentration of IV-administered therapies in the blood at various time points. These data are used to construct detailed PK profiles, which inform the development of dosing schedules that align with the pharmacological properties of the therapy.
- Vascular Interaction Studies: We evaluate the immediate interactions between therapies and the vascular endothelium and haemodynamics to understand the mechanisms behind therapy uptake or clearance. These insights are critical for anticipating and mitigating barriers to efficient delivery.
- Immune Response Monitoring: The system is equipped to detect immune responses, including the complement system and other immune cells, upon IV delivery. Continuous monitoring allows for the evaluation of immune system engagement and helps in designing strategies to avoid adverse immunogenic reactions.
Pharmacokinetics & Pharmacodynamics
- We measure the concentration of therapeutic agents within organ compartments at various time points post-administration. By correlating these levels with observed pharmacological effects, we can delineate the pharmacodynamic (PD) profile of the therapy. This analysis is crucial in understanding the dose-response relationship and therapeutic index of novel treatments.
- We study the absorption, distribution, metabolism, and excretion (ADME) properties of therapies within individual organs. This organ-specific PK information is vital for optimising dosing regimens to achieve desired therapeutic concentrations while avoiding toxicity.
- We observe and quantify the biological effects of therapies in real-time, such as enzyme inhibition, receptor activation, or gene expression changes. These PD measurements provide insights into the mechanisms of action and efficacy of the therapies.
- We conduct detailed time-course studies (up to 48hrs) that allow us to monitor the onset, peak, and duration of therapeutic effects. This information helps to understand the kinetics of the therapeutic response and guides the optimal timing and dosing of subsequent treatments.
- Leveraging our comprehensive bioanalytical tools, we screen for potential biomarkers in organ perfusates and tissues that are indicative of therapeutic action or adverse reactions.
- We integrate genomics, proteomics, metabolomics, and transcriptomics to provide a holistic view of the biological impact of therapies, leading to the discovery of novel biomarkers.
- Once potential biomarkers are identified, we validate using cross-validation methods to confirm their reliability and consistency as surrogate endpoints.
- By correlating the levels of therapies and their metabolites with changes in biomarkers, we establish pharmacodynamic relationships that can predict the dose-dependent effects and therapeutic window.
- Enzyme Activity Assays: We measure the activity of enzymes affected by the therapies to gauge their impact on metabolic pathways and organ function.
- Cell Viability and Apoptosis: We assess the impact of therapies on cell health via ATP content measurement, annexin V staining, and caspase activation.
- Clinical Endpoint Correlation: We correlate biomarker levels with clinical endpoints, enhancing the translational value of our findings and supporting their application in clinical trial design.
Ex-Vivo Informed Dosing Studies
- We conduct comprehensive dose-response studies to identify the optimal therapeutic range for each organ, maximizing therapeutic efficacy while minimizing toxicity.
- By considering unique organ characteristics, such as metabolic capacity and receptor expression profiles, we can customise dosing regimens to align with the organ’s specific biology.
- Our system evaluates how the interaction between different organs can influence the overall therapeutic index, allowing for the adjustment of dosing strategies to account for systemic effects.
- Early Toxicity Identification: With our platform’s capability to simulate human organ responses, we can identify early biomarkers of toxicity, enabling proactive adjustments to dosing regimens. We can evaluate changes in organ function, including detailed biochemical processing, to identify toxicity issues rapidly.
- Organ-Specific Toxicity Profiles: We develop detailed toxicity profiles for each organ, which inform the prediction and mitigation of organ-specific adverse effects.
- Safety Margin Assessment: By determining the safety margin – the difference between therapeutic and toxic doses – we can define safe upper limits for dosing, essential for clinical trial design.
- Multi-Organ Perfusion Studies: Our platform can simulate real-world dosing scenarios by perfusing multiple organs simultaneously, reflecting the complex interactions of whole-body pharmacokinetics.
- Dynamic Dosing Adjustments: We can dynamically adjust dosing parameters in response to immediate organ feedback, closely mirroring the clinical administration of therapies.
- Biomarker-Guided Dosing: We use identified biomarkers to guide dosing decisions, allowing for more precise and adaptive therapy administration based on individual biomarker profiles.

Acute Safety & Mechanistic Studies
- We conduct a range of cell health assays, such as cell viability, apoptosis, and necrosis detection, to evaluate the direct impact of therapies on organ cells within an acute timeframe.
- Real-time measurement of acute-phase proteins, stress response markers, and other safety-related biomarkers provides immediate feedback on the biological impact of the therapy.
- We can identify unintended pathway interferences or activations that could lead to safety concerns.

Immunological Tolerance Evaluation
- We monitor the phenotype and activation state of leukocytes within our LIVING-ORGAN system, providing insights into the immediate immunological impact of therapies and the potential for inducing tolerance.
- We are able to simultaneously quantify a broad array of up to 33 different cytokines from a single sample. This robust assay enables the detection of a comprehensive range of cytokines, encompassing both pro-inflammatory and anti-inflammatory mediators, as well as growth factors and chemokines.
- Leukocyte and cytokine data contribute to the development of an immune signature that characterises the response to therapy, which can be used to tailor treatments and mitigate adverse immune effects.
- We use advanced computational tools to analyse the interplay and regulatory networks of leukocyte phenotypes and cytokines, enhancing our understanding of the complex immune response elicited by the therapies.
- The extensive cytokine profiling data serve as a resource for discovering new biomarkers that can be used to monitor therapy response and guide personalized treatment approaches.

Metabolism Studies
- We can profile the comprehensive metabolite spectrum derived from therapeutic agents. This profiling includes identifying both expected and unexpected metabolites, providing a complete picture of metabolic diversity.
- By correlating specific metabolites with toxicity indicators, such as changes in cell viability, alterations in organ-specific biomarkers, or histopathological changes, we can deduce the potential toxic effects of therapy metabolism.
- Our platform extends beyond targeted metabolite analysis to full metabolomic profiling, which can uncover global metabolic changes induced by therapeutic agents and reveal potential metabolic biomarkers for therapy monitoring.

Virus Transduction & Cell Integration Assessment
- We measure the rate and extent of viral vector transduction in target organs by quantifying the expression of reporter genes or therapeutic genes delivered by the vectors. This is achieved through qPCR, reporter gene assays, or immunohistochemistry.
- By examining the distribution of viral vectors across different organ systems, we can assess their natural tropism, to guide improved targeting and reduce off-target transduction.
- We can evaluate the expression levels of viral receptors on cells within the organ to predict and enhance vector uptake, using techniques such as flow cytometry and immunofluorescence.
- Through longitudinal sampling and analysis, we monitor the persistence of virus/cells over time (up to 48 hrs), which is indicative of successful integration and stable transduction.
- We assess the immune response to viral vectors and transduced cells by measuring cytokine production, immune cell activation, and potential development of anti-vector antibodies.
- After viral transduction, we monitor the functional parameters of the organ to ensure that gene delivery does not impair its physiological function. This includes assessing biomarkers for organ health and conducting specific functional assays.
Molecular Biology
- Targeted Gene Sequencing: Focused sequencing of specific genes or regions to confirm the integration of therapeutic cargos.
- Transcriptome Profiling (RNA-Seq): Detailed analysis of the transcriptome to evaluate gene changes induced by therapies.
- Epigenetic Analysis: Assessment of DNA methylation and histone modifications to understand epigenetic changes influenced by therapies, which can impact gene expression and cellular behaviour.
- Gene Expression Profiling: Quantitative analysis of gene expression patterns pre- and post-therapy, providing insights into therapeutic efficacy and biological impact.
- Differential Protein Expression Analysis: Compare protein levels between samples using techniques such as 2D-DIGE (Two-Dimensional Difference Gel Electrophoresis) or mass spectrometry.
- Post-Translational Modification Analysis: Identify chemical modifications of proteins that could affect their function, using targeted mass spectrometry.
- Protein Biomarkers of Injury: Isolate and identify deleterious proteins associated with therapeutic administration.
- Enzyme Activity Assays: Measure the activity of enzymes involved in various metabolic pathways affected by CGT delivery/exposure.
- Immune Protein Analysis: Study the abundance and function of proteins involved in the immune response to CGT.
- Cytokine & Chemokine Levels: Quantify inflammatory mediators to evaluate the immune response to CGT.
- Mass Spectrometry: Characterize proteins altered by therapeutic administration, analyse changes in protein expression, and investigate signalling pathways.
- Metabolic Profiling: Analyse the complete set of metabolites present in a biological sample to gain insights into metabolic alterations due to the therapy, or response to the therapy.
- Biomarker Discovery: Identify metabolites that serve as indicators of organ health or injury.
- Metabolite Alterations: Measure the levels of specific metabolites that reflect the efficacy of the therapy, or response to the therapy.
- Organ Function Metabolic Markers: Monitor metabolites related to the function of the specific organ under treatment.
- Energy Metabolism Assessment: Evaluate the production of ATP and the overall energy status of the organ.
- Drug Metabolism & Toxicity: Assess the effects of CGT on organ metabolism and potential toxicity.
- Lipid Profile Alterations: Analyse changes in the lipid composition that may result from the efficacy of the therapy, or response to the therapy.
- Membrane Lipid Integrity: Evaluate the stability and integrity of cellular membranes by assessing the composition of membrane lipids.
- Gene Expression Changes: Use RNA sequencing or microarrays to identify alterations in gene expression patterns due to CGT exposure.
- Non-coding RNA Profiling: Examine the roles of microRNAs and other non-coding RNAs in the regulation of genes related to treatment.
- Immune Response Gene Profiling: Assess gene expression patterns related to the immune response to the therapy.
- Nucleic Acid Microarrays: Profiles gene expression across thousands of genes: Use to assess the global impact of treatment, or the response to treatment on gene expression.
- Gene Expression Profiling via RNA-Seq: Utilize next-generation sequencing to analyse gene expression changes during treatment
We can provide a streamlined service evaluating your therapy in a highly translatable model. Our platforms enable fast-tracking of innovations towards FDA submission and subsequently into clinics. For more information, please don’t hesitate to contact one of our scientists.
Pebble's Customisable
Service Package
Case Studies
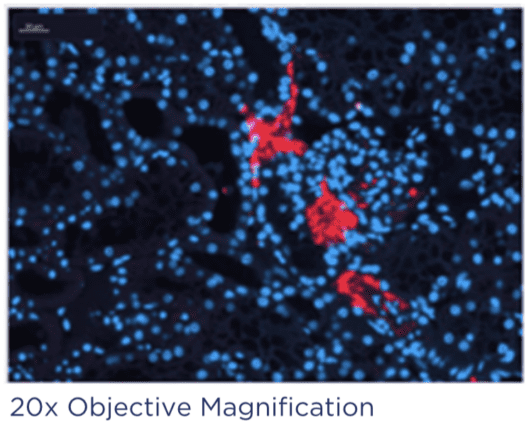
Case study 1: Safety & Biodistribution of an exosome delivery platform
Both kidneys were retrieved and randomised to exosome treatment (labelled with fluorescent dye), or control. No cargo was contained within the extracellular vesicle. An IV delivery approach was replicated.
Fluorescent staining of the samples showed deposits in the EV-treated kidney within the glomeruli.
For more information, download our white paper by signing up with your email below:
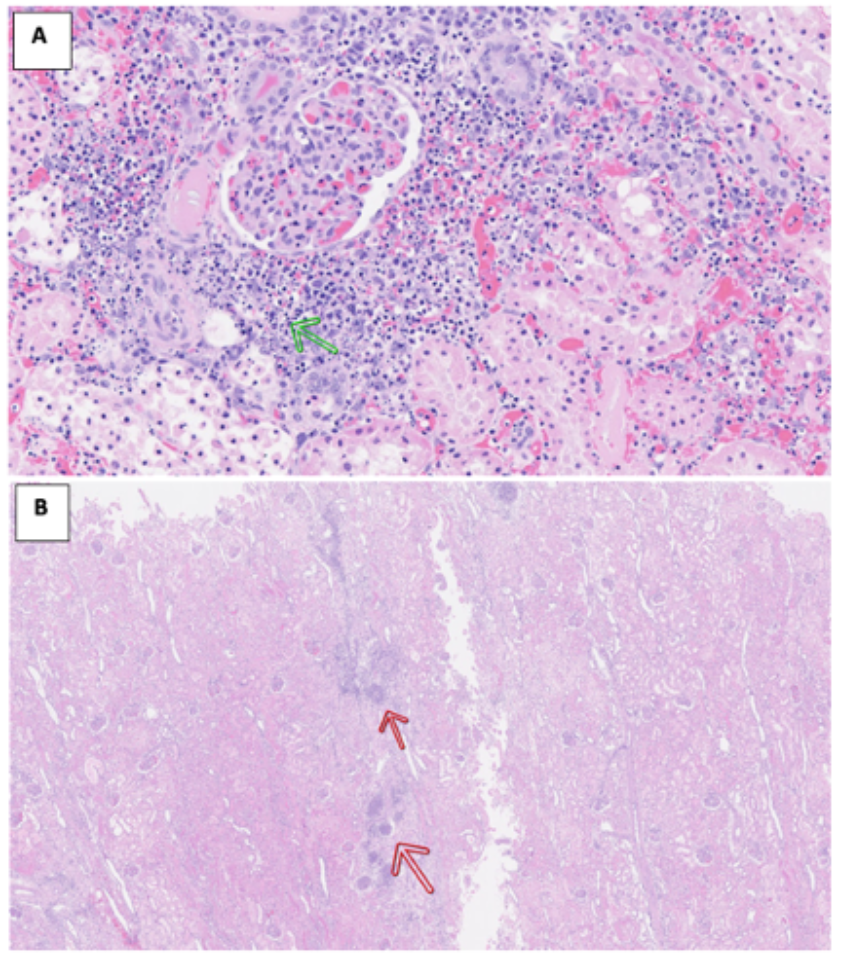
Case Study 2: Testing an AAV-based therapy on renal health
Kidneys were retrieved and randomised to AAV-treated kidney, or control. Following this, a 48-hour multi-organ liver-kidney perfusion was performed, assessing the success of delivery of therapy to the kidney when connected to a liver.
The treated kidney presented evidence of necrosis dispersed across the cortex, along with visible calyx damage. This was also seen in the multi-organ circuit. This therapy is now undergoing further pre-clinical testing.
Why Choose Pebble?
Pebble provide an extensive package, from non-reportable studies to regulatory submission ready data packages.

